Our lines of research.
The group is active in the development of new catalytic transformations that expand the merit of common organic compounds as synthetic building blocks. More specifically, along the past decade, the group gained expertise in devising chemical processes for activating unsaturated motifs relying on carbophilic catalysis, mainly based on the reactivity of gold(I), copper(I), rhodium(II) and zinc(II) catalysts. Besides, the catalytic potential associated with electrophilic forms of main group elements, particularly silicon, is also subject of study, in search for transformations that do not require the addition of transition metals.
The group has strong commitment and dedication to the education of graduate and master students. Postdoctoral colleagues and other senior researchers are also welcome.
Some representative contributions are outlined below:
A. Gold-catalyzed transformations of allenamides.
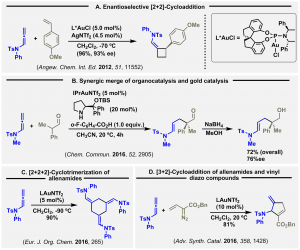
Following initial studies on [2+2] cycloaddition reactions (Adv. Synth. Catal. 2012, 354, 1651), we documented an enantioselective [2+2] catalytic process involving allenamides and vinylarenes (A, Angew. Chem. Int. Ed. 2012, 51, 11552, VIP article).
This was followed by a report on the first intermolecular reaction showing gold(I) and an organocatalyst operating in a synergistic catalytic mode (B, Chem. Commun. 2016, 52, 2905).
The group proved the viability of two additional selective transformations. Namely, the allenamide [2+2+2]-cyclotrimerization reaction (C, Eur. J. Org. 2016, 265), and the [3+2] cycloaddition involving vinyl diazo compounds. The latter implies the activation of the proximal C=C bond, rather than that of the more commonly noticed distal one of the allenamide (D, Adv. Synth. Catal. 2016, 358, 1428).
B. Zinc (and other metals) carbene generation and chemical reactivity.
The group keeps expanding the impact of its initial discoveries on zinc catalyzed transformations as a useful and complementary alternative to the addition of coinage metals to accomplish a carbophilic activation of unsaturated systems (for early work see, for instance: Angew. Chem. Int. Ed. 2013, 52, 5853, and references therein).
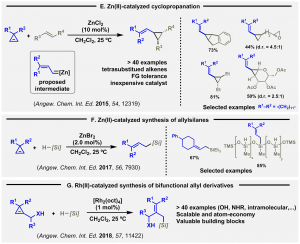
New catalytic processes triggered by in situ generation of zinc carbenes have been documented. Thus, the reaction of zinc(II) with cyclopropenes in the presence of other alkenes gives vinylcyclopropanes, in a selective manner. Interestingly, a broad range of alkenes can be used (E, Angew. Chem. Int. Ed. 2015, 54, 12139).
Then, the related zinc-catalyzed hydrosilylation reaction of in situ generated vinyl carbenes was achieved (F, Angew. Chem. Int. Ed. 2017, 56, 7930), and also the Rh (II)-catalyzed hydrosilylation reactions of demanding allyl cyclopropenols and N-tosyl cyclopropylamine derivatives (G, Angew. Chem. Int. Ed. 2018, 57, 11422).
C. Catalytic activation of heteroatom-substituted alkynes (silylalkynes and haloalkynes).
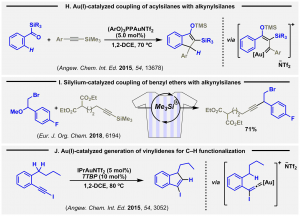
The capability of gold(I) to activate alkynyl silanes was early exploited by our group in a bis(alkynylation) reaction of aldehydes (Adv. Synth. Catal. 2013, 355, 3337; highlighted at front cover of the issue).
Subsequently, we reported the synthesis of indanones from gold-catalyzed reactions of acylsilanes with alkynylsilanes (H, Angew. Chem. Int. Ed. 2015, 54, 13678). Besides adding electrophilic gold centers to trigger thein situ release of the catalytically active silicon species, we proved that added silylium ions are competent catalytic species for the coupling of b-bromo-substituted benzyl ethers with alkynylsilanes. The products show complementary chemoselectivity to that observed in transition metal-catalyzed approaches (I, Eur. J. Org. 2018, 6194, highlighted at front cover of the issue).
The group is interested in the chemistry of gold vinylidenes. So, starting from iodo-substituted alkynes, we established stereo retentive C-H activation reaction furnishing indene scaffolds (J, Angew. Chem. Int. Ed. 2015, 54, 3052). The process involves the catalytic generation of a gold vinylidene intermediate, species ultimately responsible for the noticed activation of the benzylic C-H bond.
D. Gold-catalyzed reactions of active propargylic and benzylic positions.
We are interested in catalytic reactions of propargylic derivatives (Angew. Chem. Int. Ed. 2009, 48, 7857).
The reactivity of alkynylcyclopropanes was tested (Angew. Chem. Int. Ed. 2012, 51, 10377), and a selective synthetic manifold was recognized. Experimental work and theoretical calculations were performed to elucidate an intriguing rearrangement. A unique gold-containing allyl cation was characterized (K, Angew. Chem. Int. Ed. 2014, 53, 12097).
The catalytic reaction of 1-propargyl-1H-benzo-triazole with gold(I) complexes offers access to a-imino gold carbenes (Adv. Synth. Catal. 2016, 358, 1398). Recently, on this basis, a distinctive synthesis of 7-pyrazolylindoles was achieved (L, Adv. Synth. Catal. 2019, 361, 758).
Concerning our interest on contemporary ferrocene chemistry and its biological implications, we recently disclosed the trapping of catalytically generated o-quinone methide intermediates by metallocenes. The bioactivity of the resulting adducts was checked, offering promising results (M, Eur. J. Org. Chem. 2018, 2858; VIP article).
E. Catalytic reactions of diazocompounds.
Our group has long-standing interest in the catalytic activation of vinyl diazo compounds and its synthetic impact (for early representative work see: Adv. Synth. Catal. 2013, 355, 1848, and references therein).
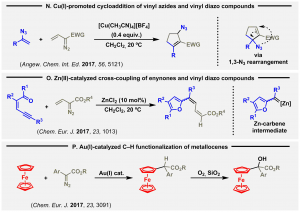
The reaction of vinyl diazo compounds with vinyl azides under the influence of catalytic copper(I) species affords cyclopentenyl allyl azides (N, Angew. Chem. Int. Ed. 2017, 56, 5121). The cyclic frame results from a copper mediated [3+2] cycloaddition, and the noticed product involves a subsequent allylic azide rearrangement step.
The group presented a detailed study on the cross coupling of vinyl diazo compounds with zinc(II) carbenes affording conjugated dienes. Now, this work significantly broadens the meaning and scope of this synthetic transformation, early discovered by SOS (O, Chem. Eur. J. 2017, 23, 1013).
The catalytic activation of vinyl diazo compounds under the influence of gold(I) complexes nicely gives rise to the functionalization of the C-H bond of metallocenes. Remarkably, a subsequent and smooth selective oxidation of the assembled benzylic C-H bond takes place under mild conditions, in a selective manner (P, Chem. Eur. J. 2017, 23, 3091, Hot Paper).