– Marín, P., Díez, F.V., Ordóñez, S. 2019. Reverse flow reactor as sustainable devices for performing exothermic reaction: Applications and engineering aspects. Chemical Engineering and Processin- Process Intensification 135, 17-189.
https://doi.org/10.1016/j.cep.2018.11.019

Abstract: Reverse flow reactors are fixed-bed reactors combining in a single intensified device chemical reaction and regenerative heat transfer (energy is stored in a bed as sensible heat). To accomplish this goal, reverse flow reactors are operated in a forced unsteady state created by periodically changing the flow direction. The most important applications of reverse flow reactor are reviewed: oxidation of hydrocarbons and sulphur dioxide, and selective catalytic reduction of nitrogen oxides. These applications involve exothermic reactions. However, recent developments have also made possible it possible the use of reverse flow reactors with endothermic reactions, such methane steam reforming. The modelling of reverse flow reactors is addressed based on the models available for fixed-bed reactors. Practical considerations regarding reverse flow reactors are considered: the selection of the type of bed, the issues of the use of lab-scale devices at dynamic conditions, the assessment of autothermal operation and heat extraction and the integrated adsorption concept. The latter is an innovative concept based on the periodical adsorption in the bed of some of the reactants, products or other feed compounds. This mass regeneration can be combined with the heat regeneration capabilities of reverse flow reactors to increase the degree of process intensification.
– Bukowski, P., Bukowska, M., Rapantova, N., Hemza, P., Niedbalska, K. 2019. Secondary Water Saturation of a Carboniferous Rock Mass in a Abandoned Mines as the Cause Behind the Changes in Geomechanical Conditions and State of Hazards in Active Mines of the Upper Silesian Coal Basin. Mine Water: Technological and Ecological Challenges 3-9.
Abstract: Mining activity, from the phase of exploration of the rock mass to the end of exploitation and abandonment of the mine, causes destruction of the rock mass and its dewatering This is the resulting in changes of the original geomechanical properties of rocks and their strengthening during and after dewatering. These changes, in turn, affect the formation and an increase in number of manifestations of the main natural hazards (fire, gas, induced seismicity and rock bursts). The current mining situation in the Upper Silesian Coal Basin (USCB) causes that the active and abandoned mines coexist in the close vicinity. The process of flooding of the abandoned mines is ongoing up to re-saturation of rocks with water. It changes the geomechanical, and thus form the hazardeous conditions. Some hazards (fire, gas, tremors and rock bursts) are limited, on the other hand among other hazards, it occurs an increase in symptoms (hazards: uncontrolled methane escapes, water, environmental, common like sinkhole, and other geomechanical ones, e.g. collapse). The reason for these changes, e.g. in the case of water hazards, may be the changes in the geomechanical properties of rocks, and conditions of safety measures, for example safety pillars or water dams. As presented in the article on the example of a safety pillar, the results of geomechanical research can be used for the design of safety measures.
Augustyniak, I., Nieldbaska, K., Bukowki, P. 2019. Use of the permeability test of Carboniferous rocks in the underground coal mines located in the Upper Silesian Coal Basin. Mine water: Technological and Ecological Challenges 559-564.
AbstractCarboniferous rock permeability tests can be used both for documenting hydrogeological and gas conditions, as well as for predicting the possibility of pre-exploitation demethanation of seams and rock mass surrounding mining excavations. The article describes the research process of rarely performed non-destructive profiling of permeability of hard rocks in different directions (x, y, z) using a PDPK-400 permeameter created by Micrometrics. The solution can be applied in mining hydrogeology, and in particular in the assessment of the water hazard in mines located close to reservoirs in flooded mine, evaluation of water filtration through safety pillars and in determining zones with increased permeability, among others for the migration of mine gases to demethanize the deposits, e.g. through holes from the surface.
– Marín, P., Vega, A., Díez, F.V., Ordóñez, S. 2020. Control of regenerative catalytic oxidizers used in coal mine ventilation air methane explotation. Process Safety and Environmental Protection 134, 333-342.
https://doi.org/10.1016/j.psep.2019.12.011
Abstract:Ventilation air methane in coal mining has an important environmental impact, since methane is a stronggreenhouse gas (1 kg of methane is equivalent to 28 kg of carbon dioxide). The oxidation of methane inregenerative oxidizers can be an attractive technique to exploit this resource. Thus, part of the heatreleased by the reaction can potentially be recovered, in addition to decreasing methane environmentalimpact. However, the concentration of methane in the mine ventilation air may change considerably withrespect to the oxidizer design value, which have negative consequences. An increase in concentration canproduce overheating (with possible damage to the unit), while a decrease in concentration may cause theextinction of the reaction. In this work, three control systems have been considered in order to deal withthese issues: proportional-integral-derivative (PID) and proportional-integral (PI) feedback controllers,and model predictive controller (MPC).The control action is based on regulating the heat extracted from the oxidizer by adjusting a hot gaspurge from the centre of the reactor. First, the control systems have been designed (i.e. the tuning parame-ters of the controller have been calculated). To carry out the design of the controllers, a simplified dynamicmodel was obtained from a complex model of the oxidizer. Then, the performance of the controlled oxi-dizer has been simulated for different types of disturbances. In these simulations, the simple PID controllerperformed well, and the MPC exhibited the fastest response.
– Ursueguía, D., Díaz, E., Ordóñez, S. 2020. Adsorption of methane and nitrogen on Basolite MOFs: Equilibrium and kinetic studies. Microporous and Mesoporous Materials 298, 110048.
https://doi.org/10.1016/j.micromeso.2020.110048

Abstract: The adsorption/desorption behaviour of methane and nitrogen on three different Metal Organic Frameworks (MOFs) was studied in this work. The objective was to obtain new insights into the adsorption process of both molecules, since the similarity in the sizes of methane and nitrogen hinders the methane recovery from lean methane-containing emissions using adsorption technologies. In that way, the capacity of adsorption of methane and nitrogen was measured on Basolite C300, Basolite F300 and Basolite A100, being remarkable the Cu interaction of the Basolite C300 with the methane, as it was deduced from both the capacity and the heat of adsorption. Evaluation of the kinetic models of adsorption leads to observe the best fitting of the Langmuir and the fractional order models. For both adsorbates, the adsorption is easier than desorption on the surface of the three materials. The main differences observed among the adsorbents were the faster adsorption of both gases on Basolite A100, as well as the larger dependence on the occupied adsorption sites. Likewise, different adsorption isotherms were used for modelling the equilibrium results obtained, Freundlich and Sips isotherms providing the best results. These facts highlight the key role of certain surface heterogeneities on the adsorption capacity. Furthermore, parameters of both the adsorption isotherms and the kinetic models could explain the differences among the adsorbents in the adsorption and desorption performance, highlighting the utility of the proposed models.
– Álvarez, M., Marín, P., Ordóñez, S. 2020. Direct oxidation of methane to methanol over Cu-zeolites at mild conditions. Molecular Catalysis 487, 110886.
https://doi.org/10.1016/j.mcat.2020.110886

Abstract: The partial oxidation of methane to methanol over a Cu-Na-MOR catalyst is studied in this work. The reaction, performed in a fixed-bed reactor, is accomplished according to a three steps cycling process: adsorption of methane, desorption of methanol promoted by water and regeneration of the catalyst. The operating conditions of the different steps of the process have been optimized to maximize methanol yield. The regeneration using air, instead of pure oxygen, has been found to increase methanol yield in the following cycle. Optimum desorption is carried out using water concentration of 5.2 mol % and 3.04 Nm3 h−1 kg−1cat. At the optimal conditions, the yield of methanol raised to 754 μmol/g Cu, corresponding to 52 % of adsorbed methane being transformed into methanol.
– Ursueguía, D., Díaz, E., Ordóñez, S. 2020. Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH4 Adsorption. Nanomaterials 10, 1089.
https://doi.org/10.3390/nano10061089
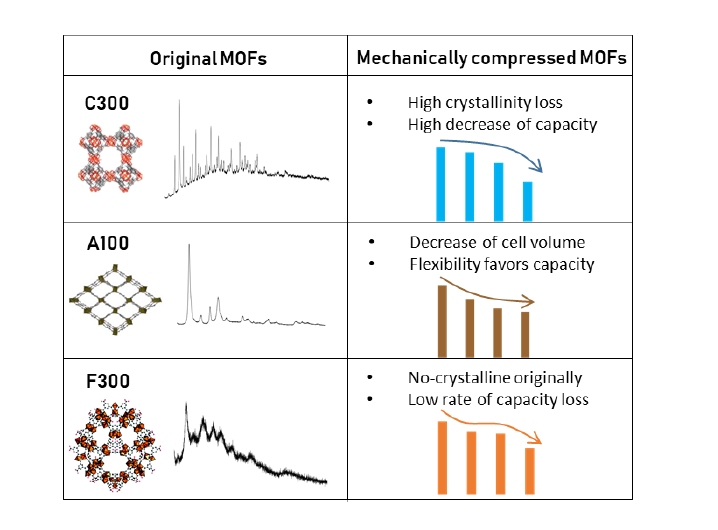
Abstract: Metal-organic frameworks’ (MOFs) adsorption potential is significantly reduced by turning the original powder into pellets or granules, a mandatory step for their use at industrial scale. Pelletization is commonly performed by mechanical compression, which often induces the amorphization or pressure-induced phase transformations. The objective of this work is the rigorous study of the impact of mechanical pressure (55.9, 111.8 and 186.3 MPa) onto three commercial materials (Basolite C300, F300 and A100). Phase transformations were determined by powder X-ray diffraction analysis, whereas morphological changes were followed by nitrogen physisorption. Methane adsorption was studied in an atmospheric fixed bed. Significant crystallinity losses were observed, even at low applied pressures (up to 69.9% for Basolite C300), whereas a structural change occurred to Basolite A100 from orthorhombic to monoclinic phases, with a high cell volume reduction (13.7%). Consequently, adsorption capacities for both methane and nitrogen were largely reduced (up to 53.6% for Basolite C300), being related to morphological changes (surface area losses). Likewise, the high concentration of metallic active centers (Basolite C300), the structural breathing (Basolite A100) and the mesopore-induced formation (Basolite F300) smooth the dramatic loss of capacity of these materials.
– Marín, P., Yang, Z., Xia, Y., Ordóñez, S. 2020. Concentration of unconventional methane resources using microporous membranes: Process assessment and scale-up. Journal of Natural Gas Science and Engineering 81, 103420.
https://doi.org/10.1016/j.jngse.2020.103420
Abstract: Unconventional methane resources are usually diluted in air, which prevents their use as feedstock in chemical or thermal processes. Some of them (e.g. coal mine ventilation air or diluted landfill biogas) are emitted directly to the atmosphere without harnessing, increasing the contribution of methane to global warming. Gas permeation membranes offer an alternative for the concentration of these methane resources, increasing considerably their harnessing possibilities. Microporous materials, such as carbon molecular sieve, zeolite or metal organic frameworks, have emerged as alternative to polymeric materials for the preparation of these membranes. The present work is based on simulations of the separation of methane and nitrogen mixtures, using SAPO-34 and carbon molecular sieve membranes. Mass transfer has been modelled in two scales: the membrane material (modelled using the Maxwell-Stefan multicomponent surface diffusion model) and the membrane module (based on the plug flow model). A sensitivity analysis of the influence of the main operating variables on the membrane performance has revealed that the most important ones are transmembrane pressure difference, methane feed concentration and membrane loading. It has been found that SAPO-34 membranes are more suited to concentrate methane in lean mixtures, while the carbon membrane perform better with rich mixtures. The membrane process has been scaled-up for a feed gas flow rate of 1000 m3/h n.t.p. with target methane recovery of 70% for two cases: lean (1%) and rich (50%) methane feed mixtures.
– Ursueguía, D., Díaz, E., Vega, A., Ordóñez, S. 2020. Methane separation from diluted mixtures by fixed bed adsorption using MOFs: Model validation and parametric studies. Separation and Purification Technology 251, 117374.
https://doi.org/10.1016/j.seppur.2020.117374
Abstract: Adsorption of methane from diluted methane/nitrogen mixtures in a fixed bed reactor was experimentally studied and modelled in this work. Three different Metal-Organic Frameworks (MOFs), Basolite C300, F300 and A100 were considered for this purpose, the adsorption bed being operated at 298 K, 0.1 MPa, and an inlet methane concentration of 2%. Methane adsorption capacities decrease in the order: Basolite C300 (0.078 mmol/g) > Basolite F300 (0.040 mmol/g) > Basolite A100 (0.028 mmol/g). In addition, a mechanistic model based on the numerical solution of an heterogeneous one-dimensional model considering axial dispersion has been used for modelling these adsorption results. Proposed model provides a reasonable fitting of the experimental fixed bed results (R2 > 0.9), using internal diffusion and axial dispersion as fitting parameters. Variation of these parameters can be explained in terms of adsorbent morphological features. Proposed model has been successfully extended to other methane adsorption processes reported in the literature, as well as to thermal desorption of methane from MOF-containing fixed bed reactors. The experimentally validated model has been used to predict the effect of main operation parameters on the performance of the MOF-based fixed beds for methane adsorption.
– Ursueguía, D., Marín, P., Díaz, E., Ordóñez, S. 2021. A nex strategy for upgrading ventilation air methane emissions combining adsorpion and combustion in a lean-gas turbine. Journal of Natural Gas Science and Engineering 88, 103808.
https://doi.org/10.1016/j.jngse.2021.103808

Abstract: This work evaluates the feasibility of harnessing a high-flow (4.4 Nm3/s) and low-concentrated (0.57% CH4) methane stream from a coal mine ventilation emission. Two consecutive processes have been coupled: a fixed bed adsorption used for methane concentration by temperature-swing adsorption, and a combustion process in a lean-gas turbine. Both processes have been simulated using rigorous mathematical model implemented in a commercial simulation package. Regarding the adsorption concentration step, optimized results showed the possibility of obtaining an outlet stream with 1.2% CH4 and a total flowrate of 3.8 Nm3/s. The gas turbine generates a net energy output of 490 kW and provides the heating required in the desorption step. The process design has been completed with an economic evaluation of the process. The estimated initial investment of the process is high (4.74 M€), and the return profitability depends a lot on the cost of the adsorbent material. The process would be profitable in a 20-year period with a 4.25% discount rate for an adsorbent cost lower than 0.6 €/kg.
– Ursueguía, D., Díaz, E., Ordóñez, S. 2021. Metal-Organic Frameworks (MOFs) as methane adsorbents: From storage to diluted coal mining streams concentration. Science of The Total Environment 790, 148211.
https://doi.org/10.1016/j.scitotenv.2021.148211

Abstract:
Ventilation Air Methane emissions (VAM) from coal mines lead to environmental concern because their high global warming potential and the loss of methane resources. VAM upgrading requires pre-concentration processes dealing with high flow rates of very diluted streams (<1% methane). Therefore, methane separation and concentration is technically challenging and has important environmental and safety concerns. Among the alternatives, adsorption on Metal-Organic Frameworks (MOFs) could be an interesting option to methane selective separation, due to its tuneable character and outstanding physical properties.
Most of the works devoted to the methane adsorption on MOFs deal with methane storage. Therefore, these works were reviewed to determine the properties governing methane-MOF interactions. In addition, the metallic ions and organic linkers roles have been identified. With these premises, decisive effects in the methane adsorption selectivity in nitrogen/methane lean mixtures have been discussed, since nitrogen is the most concentrated gas in the VAM stream, and it is very similar to methane molecule.
In order to fulfill this overview, the effect of other aspects, such as the presence of polar compounds (moisture and carbon dioxide), was also considered. In addition, engineering considerations in the operation of fixed bed adsorption units and the main challenges associated to MOFs as adsorbents were also discussed.
– Álvarez, M., Marín, P. and Ordóñez, S. 2021. Harnessing of diluted emissions by direct partial oxidation of methaneto methanol over Cu/Mordenite. Industrial and Engineering Chemistry Research 60, 9409-9417.
https://doi.org/10.1021/acs.iecr.1c01069

Abstract:
The upgrading of diluted methane emissions into valuable products can be accomplished at low temperatures (200 °C) by the direct partial oxidation of methanol over copper-exchanged zeolite catalysts. The reaction has been studied in a continuous fixed-bed reactor loaded with a Cu–mordenite catalyst, according to a three-step cyclic process: adsorption of methane, desorption of methanol, and reactivation of the catalyst. The purpose of the work is the use of methane emissions as feedstocks, which is challenging due to their low methane concentration and the presence of oxygen. Methane concentration had a marked influence on methane adsorption and methanol production (decreased from 164 μmol/g Cu for pure methane to 19 μmol/g Cu for 5% methane). The presence of oxygen, even in low concentrations (2.5%), reduced methane adsorption drastically. However, methanol production was only affected slightly (average decrease of 9%), concluding that methane adsorbed on the active centers yielding methanol is not influenced by oxygen.
– García-Vázquez, M., Marín, P. Ordóñez, S., Li, Kang., Zhang, G., García-García, F.R. 2021. Scaling up hollow fibre reactor: A study on non-PGM hollow fibre after-treatments for methane emission control under extreme conditions. Journal of Environmental Chemical Engineering 9, 106880.
https://doi.org/10.1016/j.jece.2021.106880

Abstract:
Hollow fibre-based after-treatments impregnated with iron and chromium-based oxides (non-PGM) have been tested for residual methane catalytic oxidation under extreme conditions (sulfur concentration 100-times larger than in real conditions), real timeframes (1000 h) and real operating cycles (thermal shocks). The results from this study have proven, for the first time reported in the literature, that hollow fibre reactors can be scaled up and that non-PGM impregnated hollow fibre after-treatments are the ideal candidate for the development of commercial residual methane after-treatments.
– Chaemwinyoo, U., Marín, P. Fernández Martín, C., Díez, F.V., Ordóñez, S. 2022. Assessment of an integrated adsorption-regenerative catalytic oxidation process for the harnessing of lean methane emissions. Journal of Environmental Chemical Engineering 10, 106880.
https://doi.org/10.1016/j.jece.2021.107013

Abstract:
Lean methane emissions constitute an important environmental problem due to the high global warming potential of methane compared to carbon dioxide. Typical emissions (e.g., coal mines, landfills, treatment plants, etc.) have high gas flow rate and low concentration, making its harnessing a challenging task. For this reason, an integrated adsorption/desorption and regenerative catalytic oxidation process has been proposed and designed for the treatment of an emission of 4.5 Nm3/s containing 0.20 vol% methane. To accomplish this task, methane adsorption/desorption operation has been firstly studied experimentally in a bench-scale fixed bed (adsorbent bed of 0.145 m length and 0.060 m diameter) loaded with 3 mm pellets of an activated carbon adsorbent. The information gathered on the equilibrium and kinetics, at different methane concentrations (0.20–0.50 vol%) and space velocities (WHSV 3.0–15 mol/kg h), has been used to fit a dynamic model. Then, a regenerative catalytic oxidizer has been designed and its performance studied by means of simulations. Special attention has been paid to assessing the influence of the integration of the regenerative catalytic oxidizer with the adsorption/desorption step.
