UNIT HEAD
Rosa M. Sainz
LOCATION
Laboratorio 8.11, Dpto Morfología y Biología Celular,
Facultad de Medicina,
C/ Julián Clavería, 6 33006 Oviedo
Email: sainzrosa@uniovi.es
Twitter: @bioxgroup
RESEARCH LINES
Physiological levels of Reactive Oxygen/Nitrogen Species (ROS/RNS) are part of a complex network of basic cell signaling systems. Posttranslational redox modifications in key reactive cysteine residues can alter the function of many proteins involved in crucial pathways that play a role in inflammation, cancer or aging. Our main goal is to investigate redox signaling pathways in charge of altering cell fate decisions, i.e. proliferation, differentiation and surviving mechanisms. To this purpose, our work is organized into a few areas of research focused on different aspect of Redox Biology.

1.- Involvement of redox control of tumor progression
Juan C. Mayo, Pedro Gonzalez Menéndez
By using cell, animal (murine) models or patient’s samples, we study the relevance of some of the redox-regulating proteins involved in proliferation or tumor progression. We are currently using a prostate cancer murine model (TRAMP) to assess how redox systems are altered throughout tumor progression. Combining this model with genetically modified mice expressing low and high levels of manganese-dependent, superoxide dismutase 2 (SOD2/MnSOD), namely SOD+/- or SOD2+/++, we evaluate the importance of systemic variations of this key mitochondrial redox enzyme in tumor growth. Furthermore, we also assess the oxidation/reduction of reactive cysteines in redox regulating systems as well as in cell cycle-regulating factors.
2.- Redox regulation of cell differentiation
Juan C. Mayo, Vanesa Cepas Lopez
Prostate tumor progression usually arises with an increase of neuroendocrine (NE) cells originated from an unknown source, either from epithelial transdifferentiation or from differentiation from cancer initiating cells (cancer stem cells). Upregulation of certain redox proteins confer prostate NE cells with surviving mechanisms, making them particularly resistant to antitumor agents. Our group evaluate the influence of redox signals in differentiation, in both, pathology including cancer initiating cells or epithelial tumor cells, and physiology in spermatogonial stem cells. To this aim, in addition to human tumor cell lines, we use SOD2 transgenic mice mentioned above overexpressing and downregulating this mitochondrial enzyme (SOD+/-, or SOD2+/++) in order to stablish spermatogonial primary cell cultures as well as histological and physiological studies in vivo.

3.- Cell metabolism and redox signalling
David Hevia Sánchez, Pedro González Menéndez
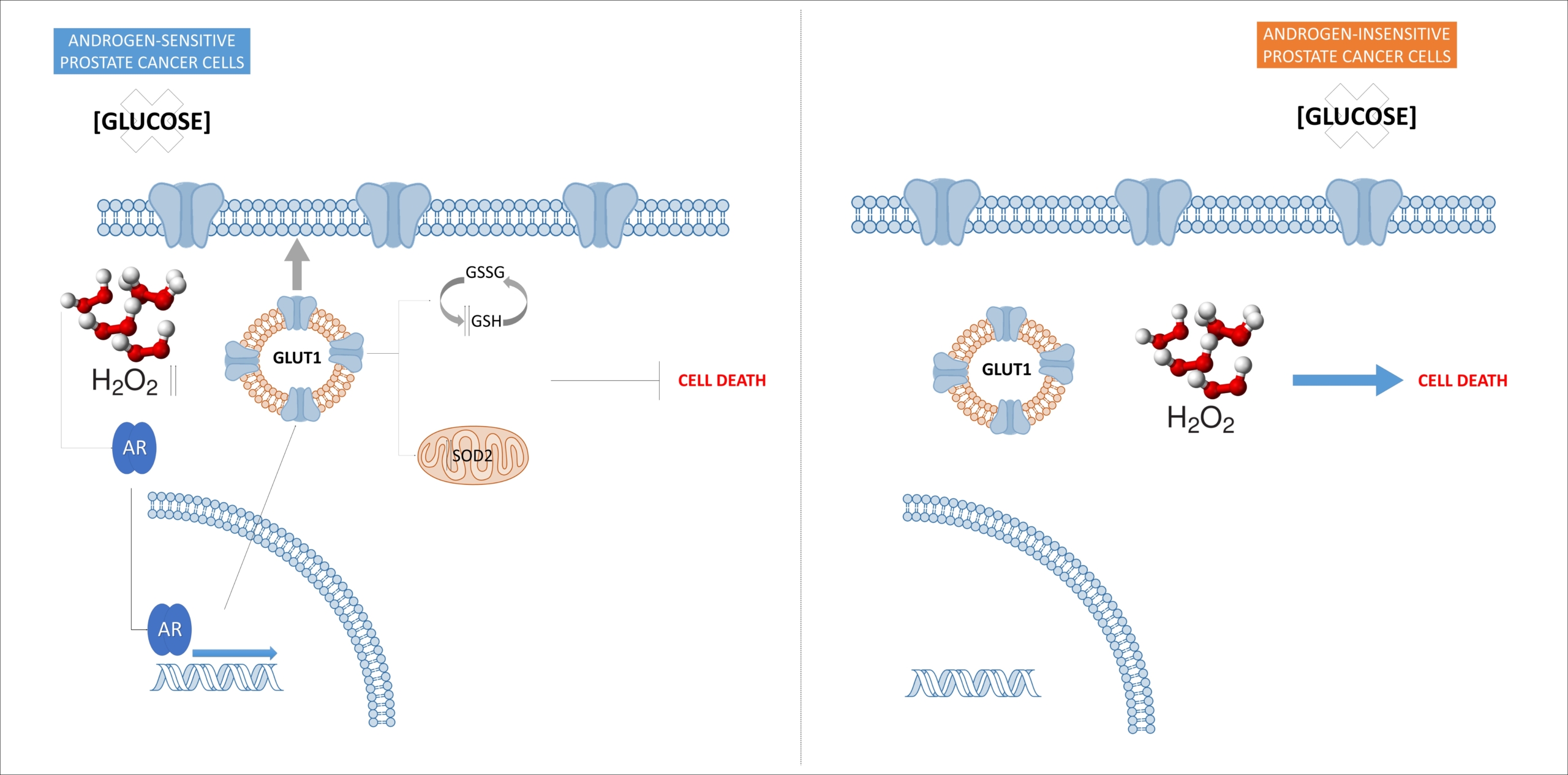
Tumor cells usually increase nutrient consumption, particularly glucose and glutamine uptake, to fulfill their high energy demand. This major metabolic switch toward aerobic glycolysis, the so called “Warburg effect”, involves a higher NADPH production through the pentose phosphate pathway (PPP) accounting for significant consequences in redox control. The levels of expression (GLUT/SLC2A), including GLUT1 or the insulin-dependent GLUT4 are altered in many types of cancer including prostate cancer. Our group is focused on the regulation of these transporters and its consequence in terms of either glucose or glutamine uptake as well as in metabolomic changes in prostate cancer cells after altering endogenous levels of redox proteins. Likewise, we are devoted to study the consequences of GLUT1 and GLUT4 overexpression in antioxidant enzyme activities and consequently in redox signaling in tumor cells.
4.- Circadian redox oscillators
Juan C. Mayo, David Hevia Sanchez
In mammals a self-regulated ‘clock gene’ system composed by different partners (CLOCK, BMAL, PERIOD O CRYP) are in charge of endogenous circadian rhythms and display a crucial role in the control of cell cycle. Recent data have shown that posttranslational hyperoxidation of specific redox proteins also follows a circadian rhythm. This regulation would include an evolutionary conserved system of mitochondrial selenoproteins in charge of hydrogen peroxide removal. Our group is particularly interested in studying the circadian control of prostate carcinogenesis, with particular attention to changes in expression and/or activity of mitochondrial selenoproteins including the Thioredoxin/Thioredoxin reductase system Trx/TrxR.
GROUP HEAD BIOGRAPHY
Rosa M Sainz obtained her PhD in 1998 at the University of Oviedo. One year later, she moved to the UTHSC at San Antonio (TX) at the laboratory of Dr. Reiter for a short-term fellow (6 months) supported by ISCIII. After coming back to Oviedo for two years (2000-01) she moved back again to San Antonio again as a post-doctoral fellow, supported by Fulbright-MEC grant.
In 2005, she joined the University of Oviedo again as a researcher under the “Ramon y Cajal” Program until 2009 and then, she reached a position as Associate Professor in the area of Cell Biology. Her thesis project was centered on the antiapoptotic properties of melatonin in thymus, which was awarded with one of the University of Oviedo Thesis Awards in 1998. Since then, her research group has been focused on the study of redox regulation of differentiation, growth and survival of tumor cells. Her research has been supported by national grants from the Health Research System (FIS-ISCIII), the Ministry of Economy (MINECO) and by local grants from the “Gobierno del Principado de Asturias”. Currently, her group collaborates with several national and international groups.
She also achieves educational activities, including both undergraduate and postgraduate degree programs, she actively participates in the master of Biomedicine and Molecular Oncology and she has been the supervisor of 5 thesis. Dra Sainz is the author of roughly 75 publications in international journals, most of them included in Q1, and has received over 6600 citations, accounting for an H index of 39 and an H 10 of 56, being part of the highly cited Spanish Scientists in the areas of Endocrinology & Metabolism and ‘Neurosciences.
SELECTED PUBLICATIONS
- Miar AB, Hevia D, Astudillo A, Velasco J, Sainz RM, Mayo JC. Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Rad Biol Med 2015 S0891-5849(15)00161-6.
- Hevia D; Gonzalez P; Quiros I; Miar A; Tan DX; Reiter R; Mayo JC; Sainz R. Melatonin uptale through glucose transporters: a new target for melatonin inhibition of cancer J Pineal Res 2015 58(2):234-50
- González-Menendez P, Hevia D, Rodriguez-Garcia A, Mayo JC, Sainz RM. Regulation of GLUT transporters by flavonoids in androgen-sensitive and insensitive prostate cancer cells. Endocrinology. 2014. 155(9):3238-50
- Hevia D, Mayo JC, Tan DX, Rodriguez-Garcia A, Sainz RM Melatonin enhances photo-oxidation of 2′,7′-dichlorodihydrofluorescein by an antioxidant reaction that renders N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK). PLoS One. 2014. 9(10):e109257
- Rodríguez-García A, Mayo JC, Hevia D, Quirós-González I, Navarro M, Sainz RM. Phenotypic changes caused by melatonin increase sensitivity to cytokines-induced apoptosis in prostate cancer cells. J Pineal Res 2013. 54(1):33-45.
- Quiros-Gonzalez I, Sainz RM, Hevia D, Mayo JC. MnSOD drives neuroendocrine differentiation, androgen independence, and cell survival in prostate cancer cells. Free Radic Biol Med. 2011. 50(4):525-36.
- Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev Camb Philos Soc. 2010. 85(3):607-23.
- Quiros I, Sainz RM, Hevia D, Garcia-Suarez O, Astudillo A, Rivas M, Mayo JC. Upregulation of manganese superoxide dismutase (SOD2) is a common pathway for neuroendocrine differentiation in prostate cancer cells. Int J Cancer. 2009. 125(7):1497-504.
- Sainz RM, Reiter RJ, Tan DX, Roldan F, Natarajan M, Quiros I, Hevia D, Rodriguez C, Mayo JC. Critical role of glutathione in melatonin enhancement of tumor necrosis factor and ionizing radiation-induced apoptosis in prostate cancer cells in vitro. J Pineal Res. 2008. 45(3):258-70.
- Hevia D, Sainz RM, Blanco D, Quirós I, Tan DX, Rodríguez C, Mayo JC. Melatonin uptake in prostate cancer cells: intracellular transport versus simple passive diffusion. J Pineal Res. 2008. 45(3):247-57.
- Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005. 165(1-2):139-49.
- Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003. 60(7):1407-26.
- Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ. Free Radic Res. 2003. 37(5):543-53

Members
| Nombre | Cargo | Teléfono | |
| Rosa María Sáinz Menéndez | Jefe de grupo | sainzrosa@uniovi.es | 985103610 |
| Juan Carlos Mayo Barrallo | Investigador | mayojuan@uniovi.es | 985102730 |
| David Hevia Sánchez | Investigador | heviadavid@uniovi.es | 985102730 |
| Isabel Quirós González | Investigador Posdoctoral | quirosisabel@uniovi.es | 985103000 Ext 5224 |
| Vanesa Cepas Lopez | Contrato Predoctoral | cepasvanesa@uniovi.es | 985103000 Ext 5224 |
| Alejandro Álvarez Artime | Contrato Predoctoral | alejandroalvarezartime@gmail.com | 985103000 Ext 5224 |
| Alba Morán Alvarez | Contrato Predoctoral | moranalba@uniovi.es | 985103000 Ext 5224 |
| Sergio Alcón Rodríguez Morán Alvarez | Estudiante de doctorado | UO251962@uniovi.es | 985103000 Ext 5224 |

