Solution Cathode Glow Discharge Optical Emission Spectroscopy
Glow Discharges operating at atmospheric pressure have shown a strong analytical potential for the fast analysis of liquids. In particular, the electrolyte-cathode atmospheric glow discharge (ELCAD) or Solution Cathode Glow Discharge (SCGD) is an alternative source for a fast elemental analysis of liquids.
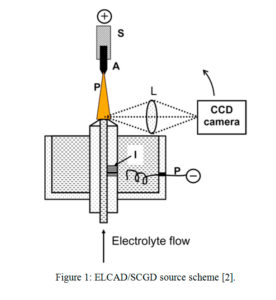
In essence, ELCAD, introduced by Cserfalvi et al.[1] in 1993, is a conically shaped GD microplasma with 3-4 mm base diameter and 3 mm height, operating between an electrolytic solution cathode and a metal (tungsten) rod anode under ~1 kV plasma voltage and 70 mA discharge current (see Figure 1). In ELCAD/SCGD, the electrolytic solution serves as the sample, just as the solid cathode does in conventional, low-pressure glow discharges. ELCAD has important advantages over conventional, nebulization-based analytical techniques, e.g., inductively coupled plasma (ICP)-OES/MS, which are extensively used as reference techniques for inorganic analysis. ICP-OES/MS requires high power (>1 kW) and gas (>15 L min-1 Ar) consumption, along with the need for vacuum equipment in the case of ICP-MS. These operating conditions effectively tie ICP-OES/MS to the laboratory. As an alternative, ELCAD has a small footprint, low power consumption (75 W), and operates in atmospheric pressure air [2].
State of the art:
Despite its simplicity, current LoD with a capillary ELCAD for a range of environmentally/industrially relevant elements (e.g., Cd, Cu, Pb, Zn) are generally in the ppb (µg/L) range. The short-term precision of the analysis is typically around 1% for ppm concentrations. However, there are certain drawbacks with ELCAD, especially, for refractory sample constituents. Therefore, new designs of portable ELCAD systems are being developed for acquiring fast, cheap and robust real-time analytical devices for a large set of elements to be operated at industrial sites, as well as deployed at outdoor sites, e.g., in mining/industrial monitoring and for wastewater monitoring [3].
Novel techniques extending Glow Discharge Spectroscopy, such as ELCAD/SCGD systems, are highly interesting for on-line quantitative analysis of fluids1) it runs at atmospheric pressure, no vacuum pumps are needed; 2) it runs in air, no noble gases are needed; 3) the liquid sample introduction does not require a nebulizer, allowing direct introduction of even liquids with particulate matter. ELCAD = Simple system for analysis of liquids. Mobile, online version of ICP-OES (1/10 of the price with minimal running costs)
Recent studies have shown ELCAD/SCGD applications involving complex samples: colloidal silica[4], tea infusions [5], mineral water [5], tuna fish [6], aquatic plant matter [6], coal fly ash [7], groundwater [7], hepatitis-B vaccine [8], lake water [9], soil leachates [9], zirconium alloys [10], simulated natural water [11], honey [12], human hair [13], and stream sediments [13]. Studies related to applications are vital for the development of the technique, but there is also a continued need for more basic research. For instance, the mechanisms involved in solution and analyte transport are not entirely understood. Recent studies have shown that additives can affect analyte signals through processes that are still not clear [14, 15]
References:
[1] T. Cserfalvi, P. Mezei, P. Apai, Emission studies on a glow discharge in atmospheric pressure air using water as a cathode, J. Phys. D: Appl. Phys., 26, (1993) 2184–2188 15
[2] P. Mezei, T. Cserfalvi, P. Hartmann, L. Bencs, The effect of OH radicals on Cr-I spectral lines emitted by DC glow discharges, SpectrochimicaActa B, 65, (2010) 218-224
[3] T. A. Doroski, A. M. King, M. P. Fritz and M. R. Webb, Solution-cathode glow discharge-optical emission spectrometry of a new design and using a compact spectrograph. J. Anal. At.Spectrom., 28(7), (2013) 1090-1095.
[4] Z. Wang, R. Gai, L. Zhou, Z. Zhang, Design modification of a solution-cathode glow discharge-atomic emission spectrometer for the determination of trace metals in TiO2,J. Anal. At. Spectrom., 29, (2014) 2042-2049.
[5] P. Jamroz, P. Pohl and W. Zyrnicki, J. Anal. At. Spectrom., 27, (2012) 1032-1037.
[6] R. Shekhar, Talanta, 93, (2012) 32-36.
[7] R. Shekhar, K. Madhavi, N.N. Meeravali and S. J. Kumar, Anal. Methods, 6, (2014) 732-740.
[8] R. Shekhar, D. Karunasagar, K. Dash and M. Ranjit, J. Anal. At. Spectrom., 25, (2010) 875-879.
[9] K. Greda, P. Jamroz and P. Pohl, Talanta, 108, (2013) 74-82.
[10] R. Manjusha, M.A. Reddy, R. Shekhar and S. J. Kumar, Anal. Methods, 6, (2014) 9850-9856.
[11] R. M. Huang, Z. L. Zhu, H. T. Zheng, Z. F. Liu, S. C. Zhang and S. H. Hu, J. Anal. At. Spectrom., 26, (2011) 1178-1182.
[12] K. Greda, P. Jamroz, A. Dzimitrowicz and P. Pohl, J. Anal. At. Spectrom., 30, (2015) 154-161.
[13] Q. Li, Z. Zhang and Z. Wang, Anal. Chim. Acta, 845, (2014) 7-14.
[14] C.G. Decker, and M. R. Webb, Measurement of sample and plasma properties in solution-cathode glow discharge and effects of organic additives on these properties. J. Anal. At.Spectrom., 31(1), (2016) 311-318.
[15] T. A. Doroski, and M. R. Webb, Signal enhancement in solution-cathode glow discharge – Optical emission spectrometry via low molecular weight organic compounds.SpectrochimicaActa – Part B Atomic Spectroscopy, 88, (2013) 40-45.